Expression of eukaryotic membrane proteins in the yeast Pichia pastoris
Certain membrane proteins can not be produced by heterologous expression in the bacterium Escherichia coli. This can be accounted for by several reasons. First, definite folding helpers (molecular "chaperones") are required for the correct insertion of eukaryotic proteins into membranes; these helper molecules are absent in Escherichia coli.
Yet the amino acid chain is synthesised in the bacterial cytoplasm. However, the proteins are not always folded into their biological conformations; regardless of their production they are either intracellularly degraded or they form larger non-structured aggregates. On the other hand, several separate post-translational modifications are required for the correct function of a eukaryotic membrane protein (e. g. glycosylation or phosphorylation). Bacterial expression systems are not proficient to carry out these actions. To this purpose often a eukaryotic expression host providing a greater biosynthetical variety is chosen, e. g. the yeast Pichia pastoris, which is capable to carry out the requested post-translational maturation reactions. Pichia pastoris (cf. the next figure) is well studied with respect to its molecular biological properties. Several examples for the production of different proteins are known, extending to the use in biotechnological applications.
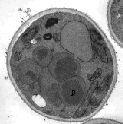
Electron micrograph of Pichia pastoris
In this project we produce a number of membrane proteins from mammals by heterologous expression using the host Pichia pastoris (GPCR's, ATPases). The proteins are extracted from the yeast. Following structural and functional studies are going on in collaboration with the group of Gerwert.

