Membrane-associated CPX-ATPases
Heavy metal translocating ATPases are integral membrane proteins that play important roles in the regulation of the intracellular concentrations of copper, zink and other metal ions. Some heavy metal ions are toxic at low concentration. Certain ATPases are employed for the detoxification of the cytoplasm; they transport the accumulated metals specifically to the outside (cf. next figure). These proteins are known to be responsible for the cellular heavy metal resistance. Various transporters (= exporters) exist that exhibit distinct specificities acting on copper, zink, lead, silver, cadmium and cobalt.
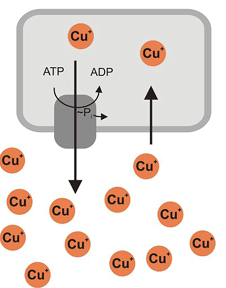
Bacterial export of copper ions
CPX-ATPases (the assignment "CPX" comes from a conserved amino acid motif (Cysteine-Proline-X, in which X can be Cysteine or any other amino acid) belong to the superfamily of the so-called P-type ATPases. They use ATP hydrolysis as source of energy to drive the metal transport (against a concentration gradient). During their catalytic reaction cycle, they form an intermediate reaction product (the so-called "phosphorylated intermediate"), namely a covalent complex of protein and phosphate (cf. "~P" in the upper figure - which relates to the designation "P-type ATPase").
The calcium ATPase is considered as a "prototype" of P-type ATPases. Its 3D structure has been solved by X-ray crystallography (cf. next figure). The sequence similarity of Ca- and CPX-ATPase is rather low, so the known structure of the former could not be used as a template for homology modelling of the latter protein.
We are working on the heterologous expression of genes coding for heavy metal translocating ATPases from various archaea and eubacteria, and on the purification of the gene products. As an expression host, we mainly use the eubacterium Escherichia coli. Our goal is to solve the structure and function of these transporter proteins.
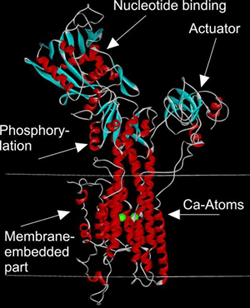
Ca-ATPase as a prototype of P-type ATPases.
(The coordinates for the display of molecular structure were taken from Toyoshima et al. (2000) Nature 405, 647-655)

